"Metabolizing Alzheimer’s:" A New Key Node in the Metabolic Network of Cognitive Decline
New Science reveals the critical role of an enzyme, IDO1, in Alzheimer's disease. We discuss these data and the implications of these findings.
What causes Alzheimer’s disease?
It’s a complex question without a linear answer. However, what I can say with confidence is Alzheimer’s is a metabolic disease characterized by dysfunctions in how neurons and their support cells, astrocytes, process and produce energy in the brain.
Chronic progressive failure in brain energy metabolism can result in dementia.
New research in Science has identified a key node in brain metabolism that acts as the convergence point of the classical amyloid and tau pathologies in the Alzheimer’s brain.
And, these new findings opens up an unexpected way to target this node to rescue brain metabolism from dementia.
(Note: if you prefer a video version of this Newsletter, scroll to the bottom)
Brain Metabolism Background
To truly understand these exciting novel findings, I need to first arm you with some brain metabolism background.
A moment ago, I mentioned “astrocytes,” a type of glial cell in the brain that support neurons. One way in which astrocytes support neurons is by providing energy substrate in the form of lactate.
Simply, astrocytes turn glucose, via glycolysis and fermentation, into lactate and then the lactate can be passed to neurons for energetic support.
Failure of astrocyte-neuron lactate transport undermines neuron’s ability to generate energy and carry out critical functions.
New Player in Brain Metabolism: IDO1
Now let’s introduce that node in brain metabolic that I mentioned, an enzyme called indolamine-2-3-dioxygenase (IDO1). IDO1 is an enzyme that converts the amino acid tryptophan into a molecule called kynurenine (KYN).
Now, what the researchers find is that the classical amyloid and tau pathologies in Alzheimer’s can each increase IDO1 activity in astrocytes, increasing KYN and thereby inhibiting astrocyte glycolysis, thus decreasing lactate transfer to neurons and starving neurons for energy.
I’ll repeat this because it’s important:
The hallmarks of Alzheimer’s, amyloid and tau, lead to activation of IDO1 activity in astrocytes.
This increases levels of the metabolite, KYN, leading to inhibition of lactate production by astrocytes.
Neurons then get less lactate transfer, leading to energetic starvation and brain metabolic failure.
Impressively, the researchers found this pathway was relevant in 3 independent mouse models of Alzheimer’s. They also found parallels in human brain cells where human astrocytes from patients with Alzheimer’s also had lower tryptophan and higher KYN levels, and that inhibiting IDO1 normalized these levels. This suggests that this pathway is active in human Alzheimer’s brain as well.
New Solutions?
It’s quite possible to inhibit IDO1 and, in so doing, restore normal astrocyte glycolysis and lactate production to feed neurons and support brain metabolism.
Indeed, the researchers found that inhibiting IDO1:
Restored astrocyte glycolysis and lactate production
Improved metabolic function
Improved neuronal function and functional markers of learning
Improved memory.
And the effects were replicated across models.
So, really, the question now is how could we attack this pathway in humans?
The answer is that there is already a cancer drug that targets IDO1, uncreatively named “PF068,” raising the possibility of repurposing cancer metabolism therapy for Alzheimer’s disease.
And what’s more, the IDO1-kynurenine pathway is also involved in other forms of neurodegenerative diseases, like Parkinson’s disease, leading to the possibility that targeting this pathway could have positive effects on dementia and neurodegenerative diseases beyond Alzheimer’s.
Prevent Cognitive Decline with Lifestyle
Now, that constitutes the thrust of what I wanted to share with you on these new data. That said, in anticipation of a question I know I’ll receive in follow-up, “What can I do to decrease my risk of Alzheimer’s disease today?” here are my high-level suggestions for protecting yourself from dementia.
Exercise
What’s good for body is good for the brain. This is in part due to indirect effects on overall health, but also due to direct effects whereby exercise can increase levels of certain neurotrophic factors like BDNF, which support brain health.
Sleep
This varies from person to person, but 7 – 9 hours is adequate for most.
Consume Long-Chain Omega-3 fatty acids, EPA and DHA
These are most easily consumed through fatty fish, like the SMASH fish: Salmon, Mackerel, Anchovies, Sardines and Herring
There may also be benefits to eating fatty fish beyond Omega-3s, including the presence of certain neuroprotective forms of selenium. If you want more on that, check out this video.
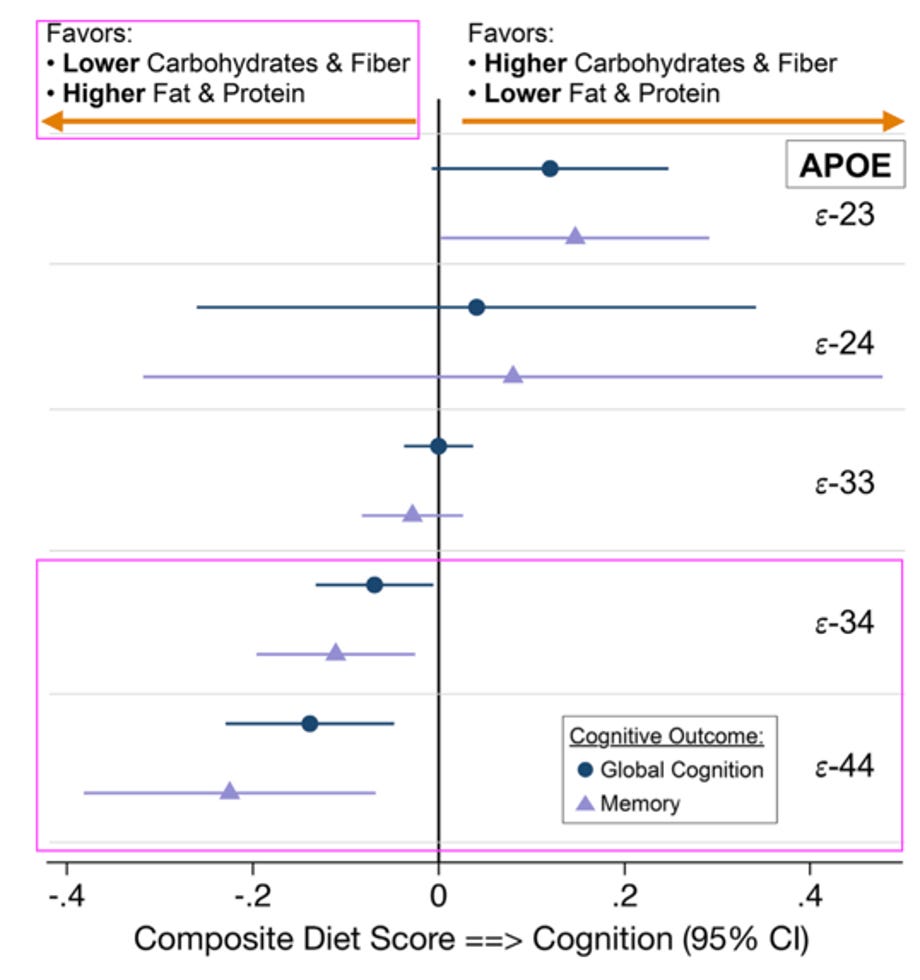
Furthermore, those with ApoE4 risk allele for Alzheimer’s, the literature suggests eating a lower carb, higher fat diet might be particularly beneficial.
Enjoy life.
While I’m no socio-neurobiologist, it appears that social connection and life purpose have powerful effects on lifespan and health span, including cognitive longevity.
So, don’t forget to enjoy the ride… it might just help you not forget.
Video version now on youtube:








I am so fascinated about metabolic and brain health... and I see every day with many patients! the one improve te other and viceversa. it is interesting to, as a curious little woman, to speculate about the why... lactate is a signaling molecule and support energy for neurons and also the production of glutamate. to much kineurine and then maybe quinolinic acid maybe are signs of alert about something going wrong (inflammation, energy scarsity, inulin resistance, infection, misfolding proteins...)... do you think over expression of IDO1 in AD is maybe a way to inhibit lactate signals and so the glutamate hyper activity and the consequent excitotoxicity? oh oh oh what a great opportunity to be her my super hero @nick
PF068 is a great name for that drug. Penny Figtree born 1968. 🩷